VELscan Assessment, DNA Ploidy Test and VELscope Vx Guided Surgery
Best News in Oral Cancer Diagnosis and Treatment
Advanced Oral (Mouth) Cancer Diagnosis and Treatment Protocol
Advanced oral (mouth) cancer diagnosis and treatment protocol is based on the latest research and advancement in technologies. The latest protocol developed consulting world’s most influential cancer research and treatment team from British Columbia Cancer Agency, Canada.
The advanced protocol shows high survival rates, better treatment outcomes, and drastically reduced recurrence rates. The protocol features the most advanced technology and encourages the best practices in the world, which is standardized abroad, but only recently made available in India.
DNA Ploidy Test and VELscope Vx Now Available in India

VELscan Assessment
DNA Ploidy Test
VELscope Vx Guided Surgery
Advancements were Anticipated for Decades
One of the biggest problem with mouth cancer was cancer detection in late-stage, when aggressive treatment is required and mortality rate is high. In this scenario patient approach doctor with well-established and clearly visible symptoms. Doctor then performs a biopsy to confirm cancer and refer patient for treatment. Following this practice, oral cancer survival rates were low and didn’t increase in last 30 years. Improvement was long-awaited.One
Better Oral Cancer Diagnosis and Treatment Protocol
Step 1. VELscan Comprehensive Oral Assessment
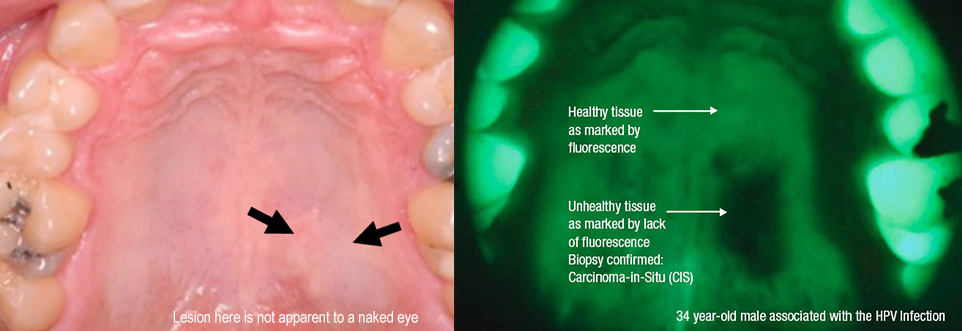
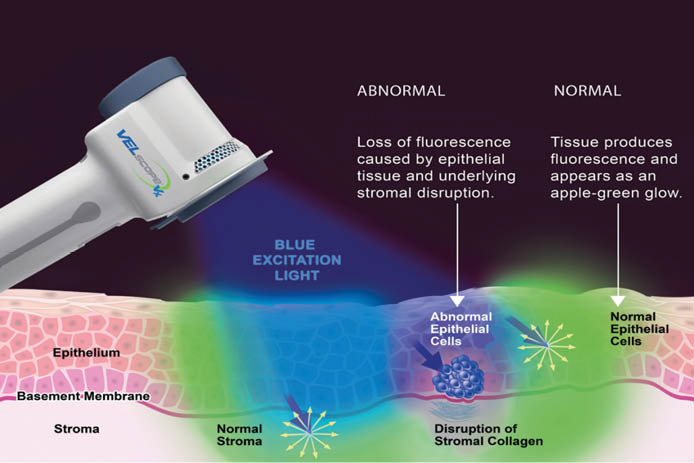
Advanced protocol starts with VELscan Assessment (using VELscope Vx based on TFV) which enhance visibility of soft tissue damage and helps to detect cancer in early stages when symptoms are not yet visible to a naked eye, or even in pre-cancerous stage (dysplasia). Furthermore, it enhances the visibility of tumor size and shape.
Step 2. DNA Ploidy Test - Diagnosis on Molecular Level
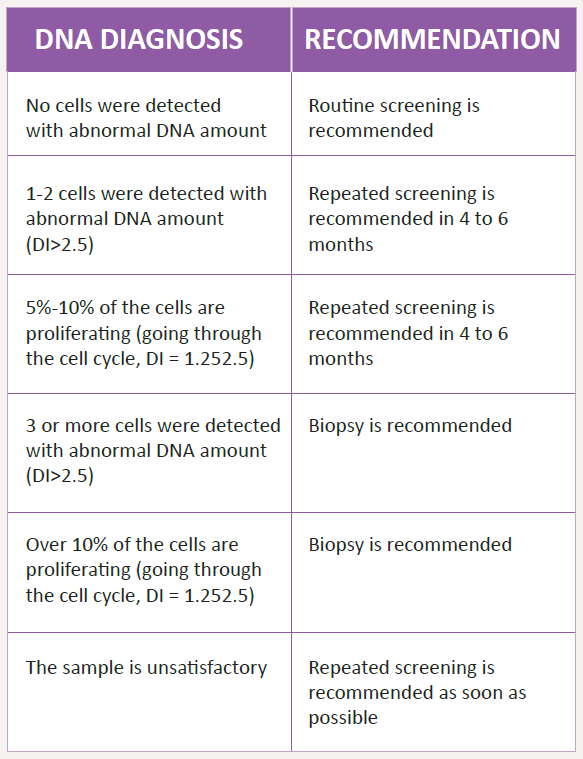
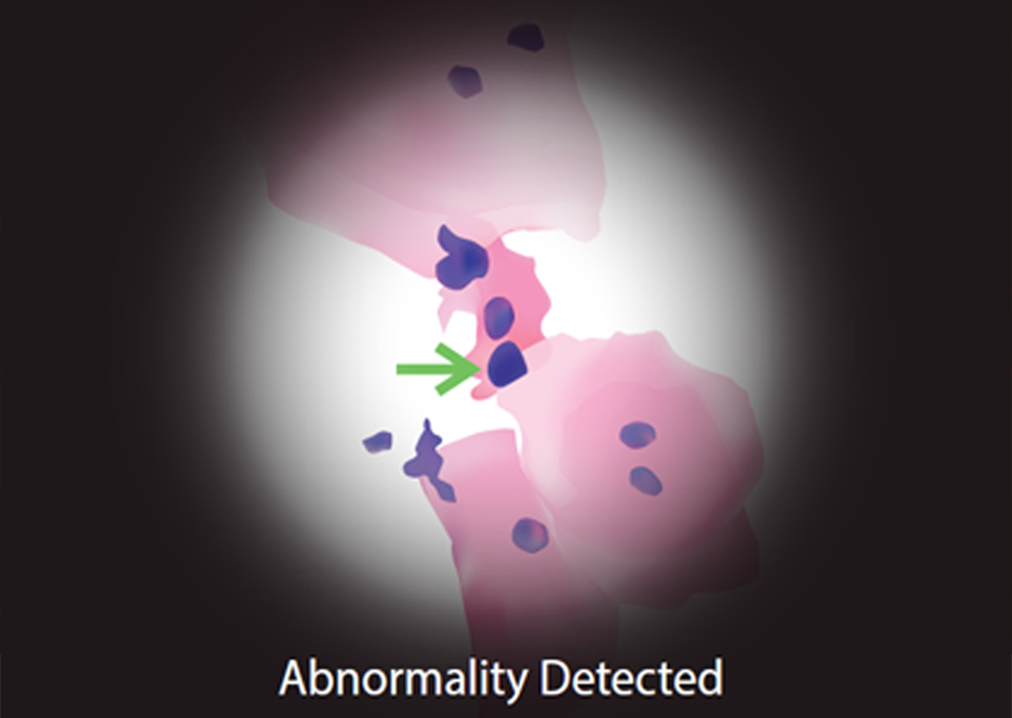
If doctor detects any suspicious abnormality in a mouth, he performs a non-invasive DNA Ploidy test. It is a painless test that collects cells from the suspicious area, then cells are analyzed on a molecular level – measuring DNA quantity. This test is the world’s most advanced and accurate test based on Artificial Intelligence (AI). Results of DNA Ploidy test shows biological behavior of cells.
The presence of cells with abnormal DNA and high activity of cells (increased cell proliferation) is the first warning signal that process of tumor development has started.
In this case, the diagnosis will be confirmed with tissue biopsy. To determine whether cancer has spread beyond the mouth imaging tests may be advised (E.g. X-ray, CT, MRI).
Step 3.Treatment Plan
Treatment for oral cancer will vary depending on the type, location, stage, and biological behavior of cancer at diagnosis. Treatment options include surgery, radiation, and chemotherapy, or a combination of treatments. To do the DNA Ploidy test before starting treatment is crucial to know the biological behavior of cells and choose the best suitable treatment plan accordingly.
DNA Ploidy report supplements pathology and imaging reports with highly accurate, objective, and quantitative results of DNA Ploidy quantity and cell activity.
Step 4.Treatment
Surgery is often the first treatment used for oral cancers. It’s most commonly used for early-stage cancers, those that haven’t spread.
The goal of the surgery is to remove a tumor with all cancerous cells, so cancer progression will be stopped. In a tumor resection, the entire tumor and a margin of healthy tissue around are removed. The margin of healthy tissue is taken out to reduce the chance of any cancer cells being left behind. However, following the old practice, it was very hard to achieve and many patients (almost 50%) had a recurrence within 3 years after surgery.
Advanced protocol – VELscope Vx guided surgery reduce recurrence rates and increase surgery success significantly. It is achieved using VELscope Vx to determine the appropriate surgical margin. The device is based on TFV that enhances the visibility of tumor size and shape. Using this advanced method removal of complete cancerous tissue can be achieved in one surgery.
Step 5. Follow Up
Most of the patients develop recurrence within 3 years after the treatment. Follow up during this period is crucial. The advanced protocol includes monitoring of cell behavior through regular DNA Ploidy testing and VELscan Assessment. DNA Ploidy testing together with VELscan Assessment using the VELscope Vx device is recommended every 6 months after surgery for 3 years after treatment or as advised by the doctor
Conclusion
VELscan Assessment, DNA Ploiy Test, and VELscope Vx Guided Surgery is the biggest improvement in mouth cancer diagnosis and treatment protocol in the last 30 years. With VELscan, DNA Ploidy test, and VELscope Vx guided surgery mouth cancer can be prevented, detected yearly, and treated successfully with high rate of survival.
WHO Recommended and FDA Approved

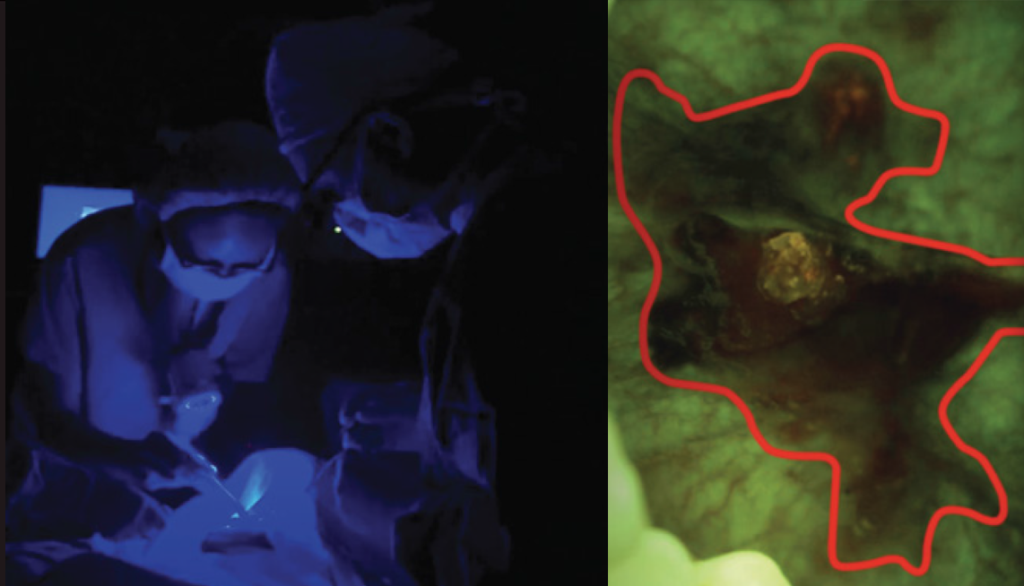


What Does All This Mean for Patients and Doctors?
1. Early disease discovery
2. DNA Ploidy site/ Biopsy site guidance
3. Determination of appropriate surgical margins around lesions to help ensure that all diseased tissue is removed.
4. Better appreciation of the full scope of mucosal involvement of particular lesions.
5. Finding difficult to detect or clinically occult satellite
lesions whether they be dysplastic or outright cancer.
6. Use of abnormal fluorescence patterns and loss of fluorescence as an aid to lesion risk assessment.
7. Photo Documentation to provide evidence-based care.
8. Better treatment outcomes and increased survival rates.
About VELscope Vx
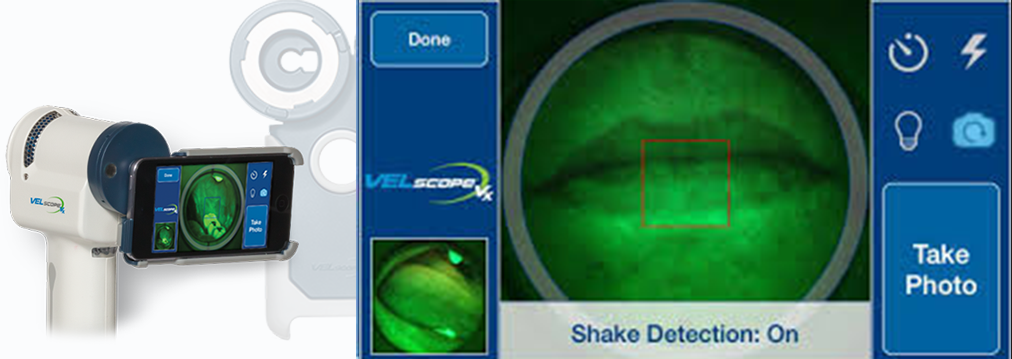
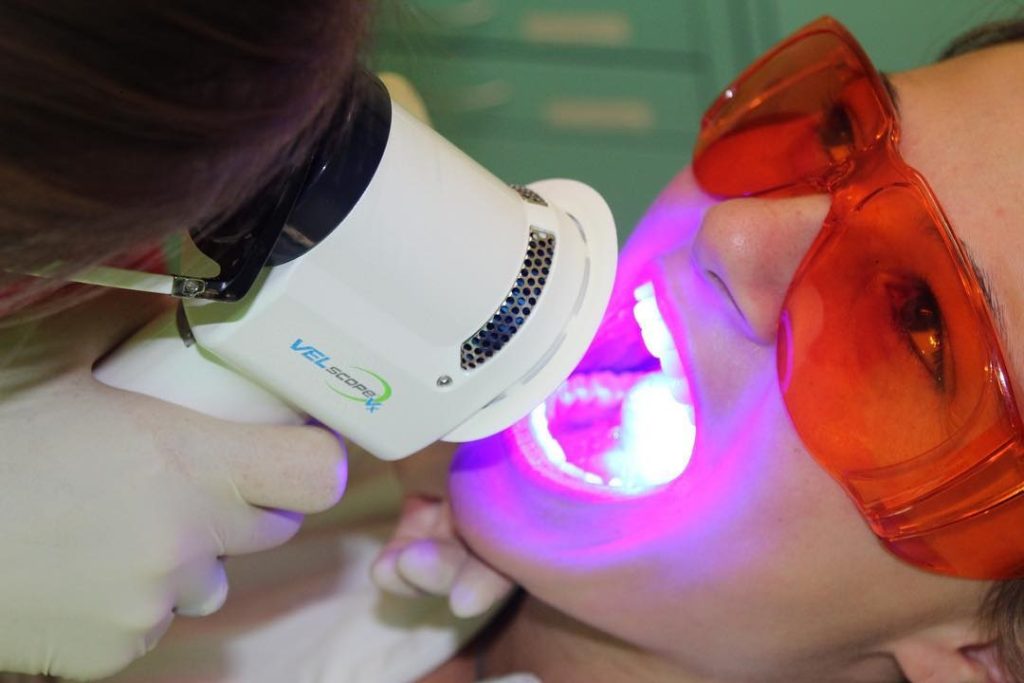
VELscope Vx Enhanced Oral Assessment System, which is used for VELscan assessment and VELscope Vx Guided Surgery is a scope that uses natural tissue fluorescence to enhance the way clinicians visualize oral mucosal abnormalities that might not be apparent or even visible to the naked eye. Recognized by the World Health Organization in 2009 as a commercialized medical device that addresses global health concerns and is accessible to low and middle-income countries, the award-winning VELscope Vx is the world’s most widely used device for the enhanced visualization of oral mucosal abnormalities. Supported by clinical studies illustrating the efficacy of the VELscope’s tissue fluorescence visualization, the device is used over 15,000 healthcare practitioners in 23 countries worldwide. The VELscope Vx is used to help detect lesions that may not be visible under traditional white light examinations, including precancerous and cancerous growths. It is further used by surgeons to help identify diseased tissue around a clinically apparent lesion and thus aid in determining the appropriate margin for surgical excision to help surgeons ensure that all diseased tissue is successfully removed when excising cancerous lesions.
- VELscope® Vx is recognized by the World Health Organization (WHO)
- VELscope® Vx is the first technology approved by the USA FDA and Health Canada for early detection of cancerous and precancerous lesions, that might be invisible to the naked eye, and to help determine the appropriate surgical margin when excision is indicated.
- This technology was developed by the British Columbia Cancer Agency and MD Anderson Cancer Center in Houston
- The technology is backed by over $50 million in research funded by the National Institutes of Health (NIH).
- The technology is backed by more clinical studies than any other device for tissue fluorescence visualization.
About DNA Ploidy Test
DNA Ploidy test is an AI-Powered quantitative diagnostic test developed by British Columbia Cancer Research. It is the world’s most advanced and accurate quantitative DNA Ploidy test which can detect aneuploidy in suspicious oral cells, up to 2 years earlier than cytology or histology alone. Aneuploidy is a proven biomarker for cancer. Every single cell in the sample is counted and the DNA quantity of every cell is measured. Furthermore, there is no place for human error, thus results are objective and accurate. DNA Ploidy test is non-invasive, painless, and well accepted by patients.
DNA Ploidy report supplements pathology and imaging reports with highly accurate, objective, and quantitative results of DNA Ploidy quantity and cell activity. DNA Ploidy report gives information necessary for accurate diagnosis and successful treatment of oral cancer.
AI-Powered DNA Ploidy Test has the highest accuracy for a
non-invasive diagnostics: Sensitivity 98% Specificity 100%
- Every cell counted
- DNA index measured
- Objective results & Highest Accuracy
- Detects abnormal cells up to 2 years earlier
- Detection in the pre-cancerous stage
- Monitors biological cell behavior
- Non-invasive sample collection
- Sample multiple sites
- Automated report generation
- Well accepted by patients
- Available for sample collection at home


Breakthrough in Oral Cancer Treatment
VELscope Vx Guided Surgery: 3-year local recurrence rate decreased from 40.6% to 6.5%.
Studies have proved that in addition to enhancing the detection of oral cancer, the VELscope system can help ensure that all targeted diseased tissue is removed during surgery and significantly reduce the recurrence of oral cancer. Exciting new research has recently appeared in the literature describing a retrospective analysis comparing patients at the British Columbia Cancer Agency who had undergone surgical excision of cancerous lesions with and without the use of fluorescence visualization guidance using the VELscope. Findings revealed that 7 of the 22 control group patients had experienced a recurrence of severe dysplasia or more serious tumors, while none of the patients who had VELscope-guided surgery experienced a recurrence of severe dysplasia or cancer. The larger study showed the results just as striking as the ones described above with only 2% of the VELscope guided surgery group presenting with severe dysplasia or worse compared to 41% for the control group.
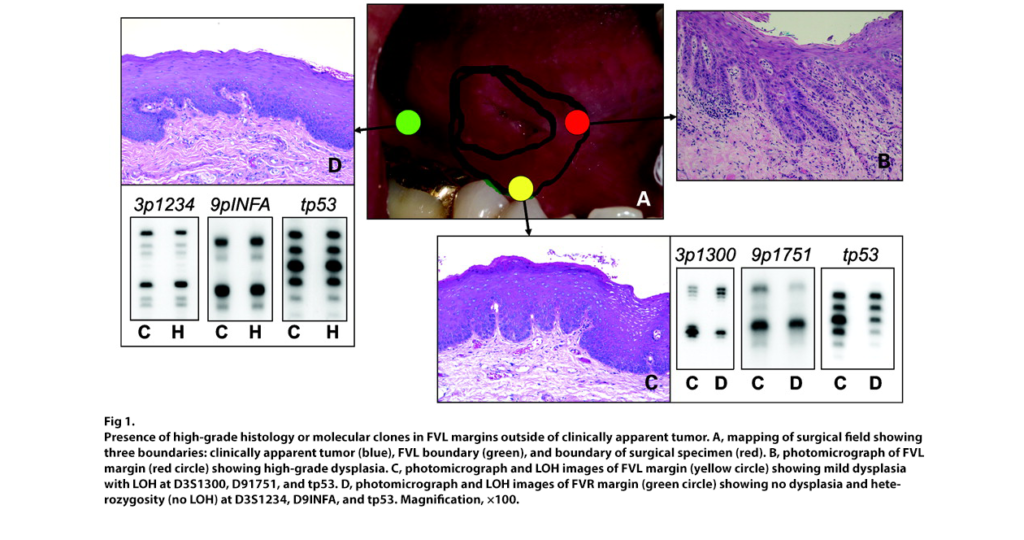
DNA Ploidy Report Supports Early Oral Cancer Diagnosis, Treatment and Recurrence Prevention
1. Pre-Surgery
DNA Ploidy test is done to identify if aneuploidy, which is proven biomarker for cancer, is present in suspicious oral tissue.
2. Post-Surgery
DNA Ploidy test is done to confirm there is no abnormal cells left in a surgical site, once surgical site is healed.
3. Routine Screening
Routine annual screening conducted to monitor biological cell behavior in order to prevent loco-regional recurrence or detect it very early.
Proven Benefits
Aneuploidy is a proven biomarker for cancer for more than 100 years.
Developed by British Columbia Cancer Research
Internationally standardized by Consensus Reports of the European Society for Analytical Celluar Pathalogy (ESACP)
Successfully commercialized in China, Canada, Germany and now India
Non-Invasive Sampling & AI-Powered DNA Analysis
Certified by:
Health Canada (Health Regulator)
Gemeinsamer Bundesausschuss (Health Regulator
Conformité Européenne (Health & Safety Regulator EEA)
China Food and Drug Administration (Health Regulator)
Central Drugs Standard Control Organization
(Indian Regulator for Diagnostic Devices)

Better Mouth Cancer Diagnosis and Treatment Protocol
Better mouth (oral) cancer diagnosis and treatment protocol is based on the latest research and advancement in technologies. The latest protocol developed consulting world’s most influential cancer research and treatment team from British Columbia Cancer Agency, Canada.
Advanced protocol shows high survival rates, better treatment outcomes, and drastically reduced recurrence rates. The protocol features the most advanced technology and encourages the best practices in the world, which is standardized abroad, but only recently made available in India.
VELscan Assessment
DNA Ploidy Test
VELscope Vx Guided Surgery
Advancements were Anticipated for Decades
One of the biggest problems with mouth cancer was cancer detection in late-stage cancer detection in late-stage, when aggressive treatment is required and mortality rate is high. In this scenario patient approach doctor with well-established and clearly visible symptoms. Doctor then performs a biopsy to confirm cancer and refer patient for treatment. Following this practice, oral cancer survival rates were low and didn’t increase in last 30 years. Improvement was long-awaited.
Better Oral Cancer Diagnosis and Treatment Protocol
Step 1. VELscan Comprehensive Oral Assessment
Advanced protocol starts with VELscan Assessment (using VELscope Vx based on TFV) which enhance visibility of soft tissue damage and helps to detect cancer in early stages when symptoms are not yet visible to a naked eye, or even in pre-cancerous stage (dysplasia). Furthermore, it enhances the visibility of tumor size and shape.

Step 2. DNA Ploidy Test - Diagnosis on Molecular Level
If doctor detects any suspicious abnormality in a mouth, he performs a non-invasive DNA Ploidy test. It is a painless test that collects cells from the suspicious area, then cells are analyzed on a molecular level – measuring DNA quantity. This test is the world’s most advanced and accurate test based on Artificial Intelligence (AI). Results of DNA Ploidy test shows biological behavior of cells.
The presence of cells with abnormal DNA and high activity of cells (increased cell proliferation) is the first warning signal that process of tumor development has started.
In this case, the diagnosis will be confirmed with tissue biopsy. To determine whether cancer has spread beyond the mouth imaging tests may be advised (E.g. X-ray, CT, MRI).

Step 3.Treatment Plan
Treatment for oral cancer will vary depending on the type, location, stage, and biological behavior of cancer at diagnosis. Treatment options include surgery, radiation, and chemotherapy, or a combination of treatments. To do the DNA Ploidy test before starting treatment is crucial to know the biological behavior of cells and choose the best suitable treatment plan accordingly.
DNA Ploidy report supplements pathology and imaging reports with highly accurate, objective, and quantitative results of DNA Ploidy quantity and cell activity.

Step 4.Treatment
Surgery is often the first treatment used for oral cancers. It’s most commonly used for early-stage cancers, those that haven’t spread.
The goal of the surgery is to remove a tumor with all cancerous cells, so cancer progression will be stopped. In a tumor resection, the entire tumor and a margin of healthy tissue around are removed. The margin of healthy tissue is taken out to reduce the chance of any cancer cells being left behind. However, following the old practice, it was very hard to achieve and many patients (almost 50%) had a recurrence within 3 years after surgery.
Advanced protocol – VELscope Vx guided surgery reduce recurrence rates and increase surgery success significantly. It is achieved using VELscope Vx to determine the appropriate surgical margin. The device is based on TFV that enhances the visibility of tumor size and shape. Using this advanced method removal of complete cancerous tissue can be achieved in one surgery.


Step 5. Follow Up
Most of the patients develop recurrence within 3 years after the treatment. Follow up during this period is crucial. The protocol includes monitoring of cell behavior through regular DNA Ploidy testing and VELscan assessment. DNA Ploidy testing together with VELscan assessment using the VELscope Vx device is recommended every 6 months after surgery for 3 years after treatment or as advised by the doctor.

Conclusion
VELscan Assessment, DNA Ploiy Test, and VELscope Vx Guided Surgery is the biggest improvement in mouth cancer diagnosis and treatment protocol in the last 30 years. With VELscan, DNA Ploidy test, and VELscope Vx guided surgery mouth cancer can be prevented, detected yearly, and treated successfully with high rate of survival.
WHO Recommended and FDA Approved

What Does All This Mean for Patients and Doctors?
1. Early disease discovery & better treatment outcomes
2. DNA Ploidy site/ Biopsy site guidance
3. Determination of appropriate surgical margins around lesions to help ensure that all diseased tissue is removed.
4. Better appreciation of the full scope of mucosal involvement of particular lesions.
5. Finding difficult to detect or clinically occult satellite
lesions whether they be dysplastic or outright cancer.
6. Use of abnormal fluorescence patterns and loss of fluorescence as an aid to lesion risk assessment.
7. Photo Documentation to provide evidence-based care.
8. Better treatment outcomes and increased survival rates.
About VELscope Vx
VELscope Vx Enhanced Oral Assessment System, which is used for VELscan assessment and VELscope Vx Guided Surgery is a scope that uses natural tissue fluorescence to enhance the way clinicians visualize oral mucosal abnormalities that might not be apparent or even visible to the naked eye. Recognized by the World Health Organization in 2009 as a commercialized medical device that addresses global health concerns and is accessible to low and middle-income countries, the award-winning VELscope Vx is the world’s most widely used device for the enhanced visualization of oral mucosal abnormalities. Supported by clinical studies illustrating the efficacy of the VELscope’s tissue fluorescence visualization, the device is used over 15,000 healthcare practitioners in 23 countries worldwide. The VELscope Vx is used to help detect lesions that may not be visible under traditional white light examinations, including precancerous and cancerous growths. It is further used by surgeons to help identify diseased tissue around a clinically apparent lesion and thus aid in determining the appropriate margin for surgical excision to help surgeons ensure that all diseased tissue is successfully removed when excising cancerous lesions.

- VELscope® Vx is recognized by the World Health Organization (WHO)
- VELscope® Vx is the first technology approved by the USA FDA and Health Canada for early detection of cancerous and precancerous lesions, that might be invisible to the naked eye, and to help determine the appropriate surgical margin when excision is indicated.
- This technology was developed by the British Columbia Cancer Agency and MD Anderson Cancer Center in Houston
- The technology is backed by over $50 million in research funded by the National Institutes of Health (NIH).
- The technology is backed by more clinical studies than any other device for tissue fluorescence visualization.

About DNA Ploidy Test
DNA Ploidy test is an AI-Powered quantitative diagnostic test developed by British Columbia Cancer Research. It is the world’s most advanced and accurate quantitative DNA Ploidy test which can detect aneuploidy in suspicious oral cells, up to 2 years earlier than cytology or histology alone. Aneuploidy is a proven biomarker for cancer. Every single cell in the sample is counted and the DNA quantity of every cell is measured. Furthermore, there is no place for human error, thus results are objective and accurate. DNA Ploidy test is non-invasive, painless, and well accepted by patients.
DNA Ploidy report supplements pathology and imaging reports with highly accurate, objective, and quantitative results of DNA Ploidy quantity and cell activity. DNA Ploidy report gives information necessary for accurate diagnosis and successful treatment of oral cancer.
AI-Powered DNA Ploidy Test has the highest accuracy for a
non-invasive diagnostics:
Sensitivity 98% Specificity 100%
- Every cell counted
- DNA index measured
- Objective results & Highest Accuracy
- Detects abnormal cells up to 2 years earlier
- Detection in the pre-cancerous stage
- Monitors biological cell behavior
- Non-invasive sample collection
- Sample multiple sites
- Automated report generation
- Well accepted by patients
- Available for sample collection at home


Breakthrough in Oral Cancer Treatment
VELscan Surgery: 3-year local recurrence rate decreased from 40.6% to 6.5%.
Studies have proved that in addition to enhancing the detection of oral cancer, the VELscope system can help ensure that all targeted diseased tissue is removed during surgery and significantly reduce the recurrence of oral cancer. Exciting new research has recently appeared in the literature describing a retrospective analysis comparing patients at the British Columbia Cancer Agency who had undergone surgical excision of cancerous lesions with and without the use of fluorescence visualization guidance using the VELscope. Findings revealed that 7 of the 22 control group patients had experienced a recurrence of severe dysplasia or more serious tumors, while none of the patients who had VELscope-guided surgery experienced a recurrence of severe dysplasia or cancer. The larger study showed the results just as striking as the ones described above with only 2% of the VELscope guided surgery group presenting with severe dysplasia or worse compared to 41% for the control group.

DNA Ploidy Report Supports Early Oral Cancer Diagnosis, Treatment and Recurrence Prevention
1. Pre-Surgery
DNA Ploidy test is done to identify if aneuploidy, which is proven biomarker for cancer, is present in suspicious oral tissue.
2. Post-Surgery
DNA Ploidy test is done to confirm there is no abnormal cells left in a surgical site, once surgical site is healed.
3. Routine Screening
Routine annual screening conducted to monitor biological cell behavior in order to prevent loco-regional recurrence or detect it very early.
Proven Benefits
- Aneuploidy is a proven biomarker for cancer for more than 100 years
- Developed by British Columbia Cancer Research
- Internationally standardized by Consensus Reports of the European Society for Analytical Celluar Pathalogy (ESACP)
- Successfully commercialized in China, Canada, Germany and now India
- Non-Invasive Sampling & AI-Powered DNA Analysis
Certified by:
Health Canada (Health Regulator)
Gemeinsamer Bundesausschuss (Health Regulator
Conformité Européenne (Health & Safety Regulator EEA)
China Food and Drug Administration (Health Regulator)
Central Drugs Standard Control Organization
(Indian Regulator for Diagnostic Devices)







CONTACT US
+91 999 044 4646
info@velscan.com
203 Varun Capital, Jangali Maharaj Rd, Pune, Maharashtra 411005